This work is done in collaboration with Dr. William H. Walker at the University of Pittsburgh.
The steroid hormone testosterone (T) regulates important quality of life determinants in men and women including bone density, cardiac health, muscle mass, prostate and reproductive tract function as well as fertility. T is essential for male fertility and targets Sertoli cells in the mammalian testis through the androgen receptor (AR) to produce the factors and environment required for germ cell development and sperm production. However, there is a lack of information regarding the cellular and molecular mechanisms by which T acts. This knowledge gap has hindered the development of therapies for infertile men and male contraception strategies as well as treatments for diseases occurring in other T-regulated tissues. It is known that T and AR can act via two mechanisms: the classical pathway in which AR directly regulates gene expression and the non-classical pathway in which T activates a series of kinases via AR. We hypothesize that each pathway regulates distinct processes that are required for male fertility and the function of other T-responsive tissues. To test this hypothesis, we will engineer and analyze transgenic mouse models lacking endogenous AR but expressing an AR mutant that selectively activates only the classical pathway or only the non-classical pathway.
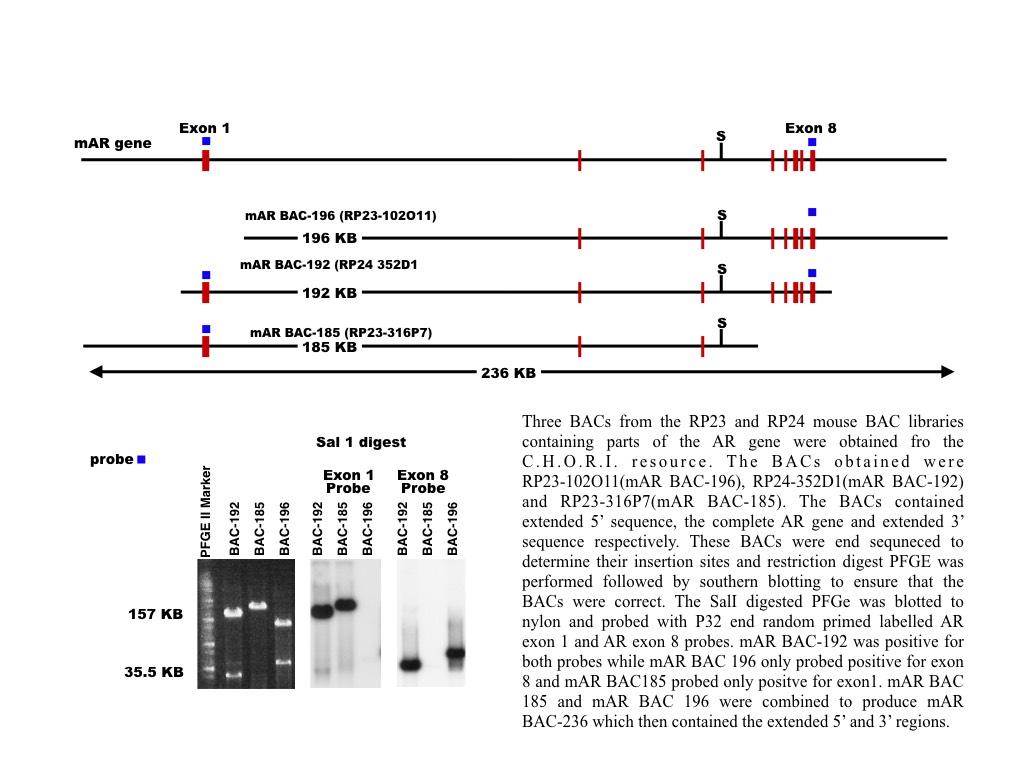
We will build our studies upon a transgenic mouse model that we created. This mouse containing a transgene encompassing the mouse genomic AR gene with extensive flanking regions and expresses the transgenic AR in T- responsive tissues while restoring fertility in mice lacking endogenous AR expression specifically in Sertoli cells. This BAC transgene was constructed by combining two BACs that overlapped in the needed sequence.
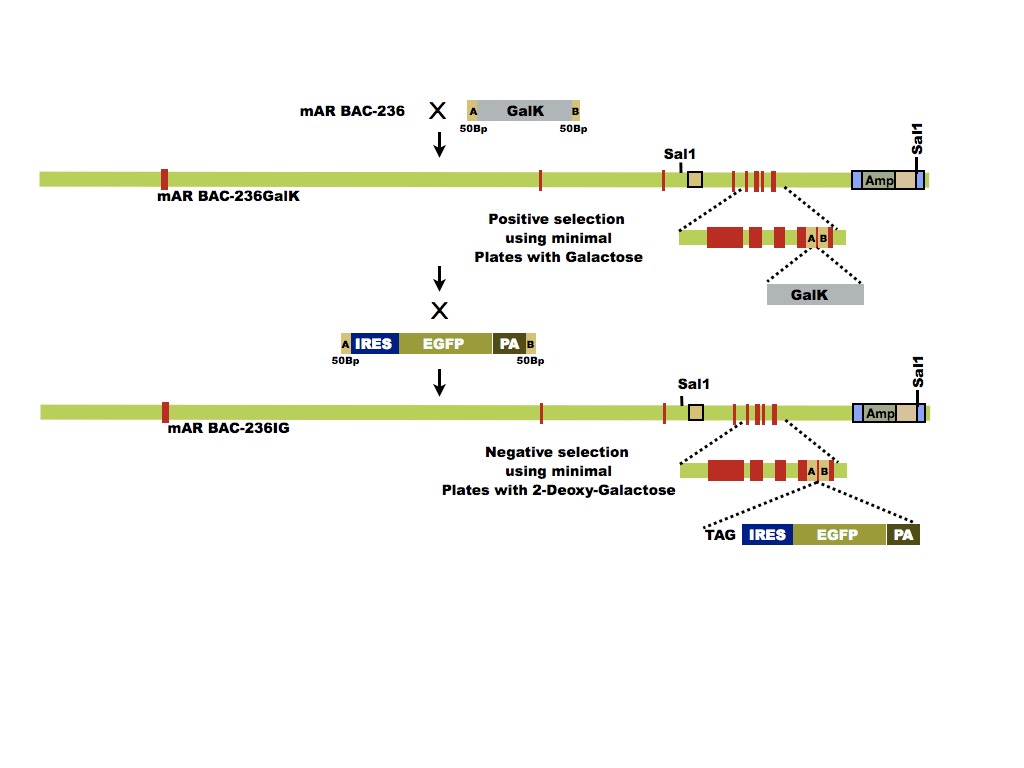
Our future aims are to recreate transgenic mice incorporating mutations in AR that will allow selective activation of only the classical or only the non-classical pathway. The transgenic mouse models will be used to create mice expressing a pathway-specific AR mutant but lacking endogenous AR expression in Sertoli cells. These mouse models will be used to determine the extent to which each pathway can support spermatogenesis and to identify the spermatogenic processes that are regulated by each pathway. These studies will permit the identification of distinct T signaling defects in male infertility patients as well as new targets for novel male contraception strategies. In the future, the mouse models also can be used to identify processes in other T- responsive tissues that are regulated by each pathway. This information could be exploited to develop therapies for diseases in T-responsive tissues including muscle wasting, bone fragility, cardiac hypertrophy and fibrosis, prostate cancer, and female infertility.